This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Creating nanoislands enhances platinum catalyst efficiency

Noble metals such as platinum can make useful catalysts to accelerate chemical reactions, particularly hydrogenation (adding hydrogen atoms to a molecule). A research team led by Professor Bruce Gates at the UC Davis Department of Chemical Engineering is interested in making platinum catalysts that are highly efficient and stable during chemical reactions.
The team's work is described in a paper in Nature Chemical Engineering and in an accompanying .
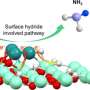
Previous work has shown that platinum arranged in clusters of a few atoms on a surface makes a better hydrogenation catalyst than either single platinum atoms, or larger nanoparticles of platinum. Unfortunately, such small clusters tend to clump easily into larger particles, losing efficiency.
Yizhen Chen, then a postdoctoral scholar in the Gates Catalysis Research Group, picked up on an idea by Jingyue Liu, now at Arizona State University, to "trap" platinum clusters on a tiny island of cerium oxide supported on a silica surface. Each island becomes its own chemical reactor.
Chen, Gates and colleagues were able to show that they could produce these clusters, that they showed good catalytic activity in hydrogenation of ethylene, and that they were stable under severe reaction conditions.
These confined metal clusters could provide a new route to produce stable catalysts for the chemical industry.
More information: Paper: Yizhen Chen et al, Stabilizing supported atom-precise low-nuclearity platinum cluster catalysts by nanoscale confinement, Nature Chemical Engineering (2025).
Research Brief: Nanoscale confinement strategy for the stabilization of few-atom platinum cluster catalysts, Nature Chemical Engineering (2025).
Journal information: Nature Chemical Engineering
Provided by UC Davis