This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
A strategy of ligand-protected direct hydrogen reduction to prepare bimetallic cluster catalysts

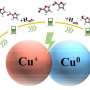
Researchers have developed a ligand-protected direct hydrogen reduction strategy to prepare zeolite-confined Pt-Pd bimetallic cluster catalysts. These catalysts efficiently facilitate hydrogen production from ammonia borane (AB) solvolysis and the tandem hydrogenation of nitroarenes. In this tandem reaction, AB undergoes hydrolysis at the platinum sites to generate active hydrogen species, which are then transferred to neighboring palladium sites to reduce nitroarenes.
The findings are in the journal Science China Chemistry. The study was led by Prof. Ning Wang (College of Chemistry and Chemical Engineering, Qingdao University) and Prof. Qiming Sun (College of Chemistry, Chemical Engineering and Materials Science, Soochow University).
Hydrogen production from the hydrolysis or methanolysis of AB and the selective hydrogenation of nitroarenes are critical reactions in hydrogen energy storage, transportation, utilization, and value-added chemical synthesis. Supported metal catalysts are widely used in these processes; however, the high surface energy of metal nanoparticles often results in issues such as aggregation or leaching of metal species.
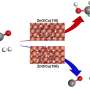
Zeolites, with well-defined nanoporous structures and excellent thermal stability, are considered ideal supports for confining ultrasmall metal particles, providing a unique and effective solution for the development of highly active and stable catalytic materials.
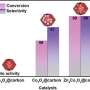
"The confinement effect of the zeolite channels endows the Pt-Pd@S-1 catalysts with exceptional catalytic activity and stability in AB solvolysis. Additionally, the bimetallic catalytic system significantly enhances O-H bond cleavage, thereby increasing the rate of hydrogen production from AB hydrolysis and methanolysis.
"Notably, in the tandem reaction of AB hydrolysis and nitroarene hydrogenation, the Pt and Pd active sites are responsible for AB hydrolysis and nitroarene hydrogenation, respectively, demonstrating outstanding catalytic activity and shape-selective performance under low temperature and atmospheric pressure," Sun says.
More information: Jiafu Li et al, Subnanometric bimetallic Pt–Pd clusters in zeolites for efficient hydrogen production and selective tandem hydrogenation of nitroarenes, Science China Chemistry (2024).
Provided by Science China Press