Team discloses the formation of burning ice in oceanic clay rich sediment

A KAIST research team has identified the formation of natural gas hydrates, so-called flammable ice, formed in oceans. Professor Tae-Hyuk Kwon from the Department of Civil & Environmental Engineering and his team found that clay minerals in oceanic clay-rich sedimentary deposits promote formation of gas hydrates and proposed the principle of gas hydrate formation in the clayey sedimentary layers.
Gas hydrates are ice-like crystalline structures composed of hydrogen-bonded water molecules encapsulating gas molecules. They are also known as burning ice. Deposits are so huge that researchers are exploring them as an alternative energy source. Conventionally, it was believed that formation of gas hydrates is limited in clay sedimentary deposits; however, the unexpected abundance of natural gas hydrates in oceanic clay-rich sedimentary deposits raised the issue of how they formed.
The surfaces of natural clay minerals are negatively charged, and thus unavoidably generate physicochemical interactions between clay and water. Such clay-water interactions have a critical role in the occurrence of natural gas hydrates in clay-rich sedimentary formations.
However, researchers encountered difficulty in analyzing hydrate formation because of the cations contained in clay particles, which balance the clay surface charges. Therefore, clay particles inevitably release the cations when mixed with water, which complicates the interpretation of experimental results.
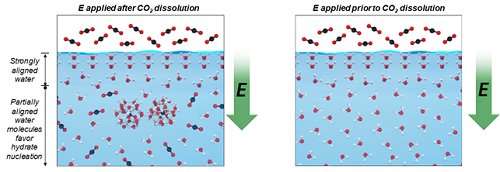
To overcome this limitation, the team polarized water molecules with an electric field and monitored the induction times of water molecules forming gas hydrates. They found that the 10 kV/m of electric field promoted gas hydrate nucleation under certain conditions rather than slowing it down, due to the partial breakage of the hydrogen-bonded water clusters and the lowered thermal energy of water molecules.
Professor Kwon said, "Through this research, we gained better insight into the origin of gas hydrates occurrence in clay-rich sedimentary deposits. In the near future, we will soon be able to commercially produce methane gas from natural gas hydrate deposits."
This research, led by PhD candidate Taehyung Park, was published online in Environmental Science and Technology on February 3.
More information: Taehyung Park et al, Effect of Electric Field on Gas Hydrate Nucleation Kinetics: Evidence for the Enhanced Kinetics of Hydrate Nucleation by Negatively Charged Clay Surfaces, Environmental Science & Technology (2018).
Journal information: Environmental Science & Technology , Environmental Science and Technology