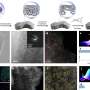
Volcano plot showing the relationship between dynamic dz2 orbital occupation and CO2-to-CO conversion rate. Credit: National Taiwan University
A research team at National Taiwan University has discovered how single metal atoms dynamically adapt their structure and electronic configuration during electrochemical reactions, dramatically improving the efficiency of carbon dioxide (CO2) conversion.
Using real-time operando X-ray techniques, the scientists tracked how these atoms behave under working conditions and revealed a key electronic signature linked to their catalytic performance. The study was in the Journal of the American Chemical Society.
The study focuses on atomically dispersed transition metal-nitrogen-carbon catalysts—materials that host individual atoms of metals like nickel (Ni), iron (Fe), and manganese (Mn) within nitrogen-doped carbon frameworks. These catalysts are known for their high efficiency in converting CO2 to carbon monoxide (CO), a valuable industrial feedstock for producing fuels and chemicals.
What sets this study apart is its use of operando time-resolved X-ray absorption spectroscopy, which allowed the researchers to directly observe changes in both the geometric and electronic structures of the catalysts as the reaction occurred.
The findings reveal that a specific electronic state—a half-filled dz2 orbital on the central metal atom—is a key factor for high catalytic activity. This orbital enables optimal binding with CO2 reaction intermediates, leading to faster and more selective CO production.
Among all the tested catalysts, nickel emerged as the most efficient. Under operating conditions, Ni atoms shifted into a square-pyramidal configuration, stabilizing the desirable half-filled dz2 state. In contrast, metals with fully filled or empty dz2 orbitals, such as copper and zinc, showed significantly lower performance.
This work not only deepens our understanding of catalytic mechanisms at the atomic scale but also points the way toward more efficient carbon recycling systems. By identifying clear structure-activity relationships, the findings offer a roadmap for developing high-performance, low-cost catalysts to help tackle global carbon emissions.
"This study provides the first real-time observation of how single atoms dynamically adapt to their environment to enhance CO2 conversion, and it opens a new path for designing next-generation catalysts based on orbital-level engineering," said Prof. Hao-Ming Chen.
More information: Jiali Wang et al, Adapting Atomic Configuration Steers Dynamic Half-Occupied State for Efficient CO2 Electroreduction to CO, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by National Taiwan University