This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study introduces lead-coated nickel catalyst for enhanced hydrogen evolution reaction efficiency
In a recent collaboration, a research team developed a hydrogen evolution reaction catalyst that minimizes degradation caused by reverse current in alkaline water electrolysis systems.
The team consists of Professor Yong-Tae Kim, Dr. Sang-Mun Jung, and Yoona Kim, an MSc, from the Department of Materials Science and Engineering at Pohang University of Science and Technology (POSTECH) and is led by Professor Jeong Woo Han from Seoul National University. Their research was as a cover paper in the journal Advanced Functional Materials on July 3.
The electricity generated from the renewable energy sources such as solar, wind, hydro, and geothermal are not constant; it fluctuates with weather and climate conditions. To harness and utilize this energy, it must be reliably stored and delivered to the grid, and hydrogen plays a crucial role in this process.
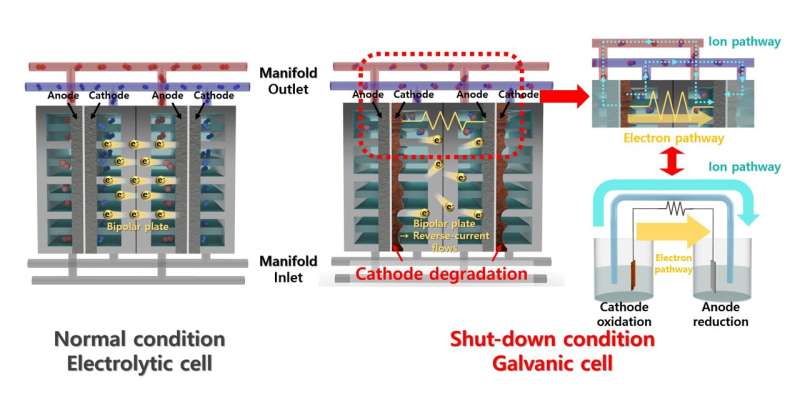
A common method for producing hydrogen is the water electrolysis system, which generates hydrogen by electrolyzing water. The alkaline water electrolysis system (AWE), which utilizes an alkaline solution, offers advantages such as relatively low cost and high durability. However, the intermittent energy supply can lead to degradation of the electrolyzer. When power is not supplied, the reverse current can damage the electrodes and reduce their durability.
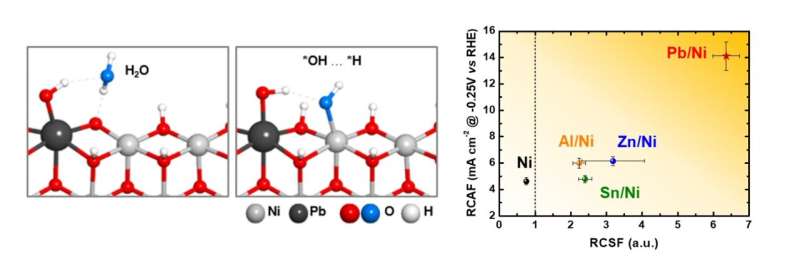
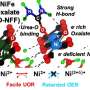
To address this issue, the research team introduced a lead (Pb) coating on a nickel (Ni) catalyst. Although lead is typically not used as a catalyst due to its low activity in hydrogen evolution reactions, the study found that coating nickel, a hydrogen evolution reaction catalyst, with lead enhances its performance. The lead acts as a co-catalyst, promoting both proton desorption and water dissociation, thus increasing the efficiency of hydrogen evolution reaction.
Additionally, the research team demonstrated that the catalyst has a strong resistance to reverse current through the repetitive oxidation reactions when the AWE was repeatedly started and stopped. Unlike previous catalysts that required additional equipment to address the reverse current issue in AWEs, this newly developed catalyst enhances hydrogen evolution reaction efficiency with just a lead coating and resists reverse current simultaneously.
Professor Yong-Tae Kim of POSTECH who led the research remarked, "This is the first study to address the degradation caused by reverse current in AWEs with a material solution. We hope that this research will improve the durability of AWEs and advance the era of a green hydrogen economy."
More information: Sang鈥怣un Jung et al, Reverse鈥怌urrent Tolerance for Hydrogen Evolution Reaction Activity of Lead鈥怐ecorated Nickel Catalysts in Zero鈥怗ap Alkaline Water Electrolysis Systems (Adv. Funct. Mater. 27/2024), Advanced Functional Materials (2024).
Journal information: Advanced Functional Materials
Provided by Pohang University of Science and Technology