This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research team introduces superaerophobic three-dimensional nickel catalysts for accelerated water electrolysis
Water electrolysis process is a system that produces hydrogen by electrolyzing water. It is an eco-friendly technology that can produce hydrogen fuel, a future energy source, without emitting environmental pollutants, but its limitations include low hydrogen production efficiency and high production costs. A team of researchers from Pohang University of Science and Technology (POSTECH) has published in Advanced Materials that solved both problems at once.
A collaborative research team comprising Professor Jong Kyu Kim, Jaerim Kim, a Ph. D. candidate, Professor Yong-Tae Kim, and Doctor Sang-Mun Jung from the Department of Materials Science and Engineering at the POSTECH has succeeded in developing an economical and efficient water electrolysis catalyst that overcomes the limitations of conventional catalysts by using an oblique angle deposition method and nickel (Ni).
The water-electrolysis processes employ costly precious metals like platinum as catalysts for hydrogen production, rendering the process excessively costly. Furthermore, the use of conventional thin-film catalysts often results in inadequate separation of hydrogen bubbles, leading to blockages in the catalyst's active sites or hindering reactant movement, ultimately diminishing process efficiency.
In response to these challenges, the research team opted for oblique angle deposition and nickel. This technique involves tilting the substrate during deposition to easily create diverse nanostructures of the material, offering a straightforward and inexpensive solution. Moreover, nickel stands out as an abundant non-precious metal catalyst on Earth, demonstrating relatively high efficiency in hydrogen generation.
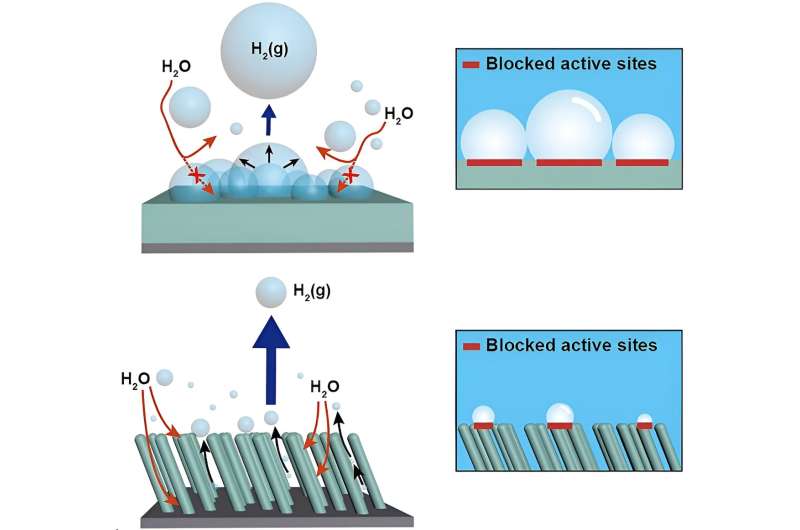
The team utilized an oblique angle deposition method to synthesize nickel featuring finely crafted, vertically aligned nanorods protrusions. In contrast to conventional nanostructures that merely augment the catalyst's surface area, the researchers engineered highly porous nickel nanorods array, presenting a unique superaerophobic surface properties to solve the hydrogen adherence issues.
Experimental results revealed that hydrogen bubbles generated during the electrolysis process exhibited the accelerated separation of hydrogen bubbles from the superaerophobic surface. The team's superaerophobic three-dimensional nickel nanorods catalyst, with effective pore channels, demonstrated a remarkable 55-fold improvement in hydrogen production efficiency compared to an equivalent amount of nickel in a traditional thin film structure.
Professor Jong Kyu Kim and Ph. D. Jaerim Kim, leading the research, explained, "By enhancing the efficiency of the water electrolysis process for green hydrogen production, we are advancing towards a hydrogen economy and a carbon-neutral society. This breakthrough not only benefits water electrolysis but also holds promise for various other renewable energy applications where surface reactions play a crucial role, such as carbon dioxide reduction and light energy conversion systems."
More information: Jaerim Kim et al, Efficient Alkaline Hydrogen Evolution Reaction Using Superaerophobic Ni Nanoarrays with Accelerated H2 Bubble Release, Advanced Materials (2023).
Journal information: Advanced Materials
Provided by Pohang University of Science and Technology