This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Temperature-driven epigenetic reprogramming lets plants control cell fate and adapt to environmental change

A team of researchers led by Prof. Xiao Jun from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences has discovered a new way in which temperature can influence the fate of plant cells by regulating epigenetic marks.
Their findings, online in Developmental Cell on April 22, focus on how temperature alters key epigenetic signals—chemical tags on DNA-packaging proteins that determine which genes are active and which are silent. This work provides a powerful example of how nature and nurture interact—underscoring how temperature can modulate cell identity.
In the model plant Arabidopsis thaliana, the transition from seed to seedling involves turning off a specific set of genes that are only needed during embryonic development. These genes must be silenced for the plant to proceed with normal growth.
To accomplish this, the plant relies on two groups of proteins that silence gene expression: the Polycomb repressive complexes PRC1 and PRC2. These complexes modify the structure of chromatin—the DNA and proteins that make up chromosomes—by adding chemical marks that make it harder for genes to be read and activated.
Specifically, PRC2 adds a well-known repressive tag called H3K27me3 to histone proteins; while PRC1 adds related tags, H2Aub or H2A.Zub, to the histone H2A or a variant histone called H2A.Z, respectively. Together, these tags create a repressive chromatin environment that locks down embryonic genes, ensuring they remain off after germination.
Dysfunction of PRC1 or PRC2, however, relieves the inhibition of key embryonic genes (e.g., LEC1, ABI3) and flattens the epigenetic landscape, thereby leading to re-acquisition of embryonic identity and de-differentiation into callus in Arabidopsis, reflecting Waddington's concept of developmental plasticity.
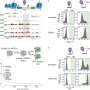
Remarkably, the researchers found that low ambient temperatures (16℃) can partially compensate for PRC2 loss, rescuing developmental defects in prc2 mutants. By integrating transcriptomic, epigenomic, and genetic analyses, they identified the transcription factor TOE1 as a key mediator of this temperature response.
Under normal conditions, TOE1 directly interacts with the INO80-C chromatin remodeling complex to remove H2A.Z from nucleosomes at embryonic gene loci, thus promoting the transition from embryonic to post-germination growth. However, at low temperatures, TOE1 expression is reduced. As a result, H2A.Z is no longer efficiently removed from these genes and begins to accumulate.
This accumulation creates an opportunity for PRC1 to attach a repressive tag to leftover H2A.Z thus converting it into H2A.Zub. This modified form of H2A.Z now serves as a silencing signal, helping to repress the very embryonic genes that PRC2 would normally control. In this way, low temperature compensates for the absence of PRC2 and restores at least part of the necessary repression machinery.
This research provides a new strategy for optimizing callus differentiation by adjusting culture temperature to enhance crop regeneration. It also highlights the broader significance of H3K27me3—the highly conserved eukaryotic epigenetic marker added by PRC2.
Its conservation in animals and plants suggests its key role in the process of multicellular differentiation: In plants, it balances developmental plasticity and environmental adaptability; in animals, it maintains somatic cell identity and prevents de-differentiation. In humans, H3K27me3 dysregulation in cancer can reactivate stem-like properties, mirroring callus formation in plants.
Understanding the mechanisms of low temperature-induced epigenetic reprogramming may therefore inform cancer treatments. All in all, this research shows that the genome isn't a fixed instruction manual—it is more like a living negotiation between environmental inputs and epigenetic controls.
More information: Kehui Zhu et al, Dynamic control of H2A.Zub and H3K27me3 by ambient temperature during cell fate determination in Arabidopsis, Developmental Cell (2025).
Journal information: Developmental Cell
Provided by Chinese Academy of Sciences