This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Atmospheric reaction between Criegee intermediates and water found to be unexpectedly fast

Criegee intermediates (CIs)—highly reactive species formed when ozone reacts with alkenes in the atmosphere—play a crucial role in generating hydroxyl radicals (the atmosphere's "cleansing agents") and aerosols that impact climate and air quality. The syn-CH3CHOO is particularly important among these intermediates, accounting for 25%–79% of all CIs depending on the season.
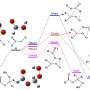
Until now, scientists have believed that syn-CH3CHOO primarily disappeared through self-decomposition. However, in a study in Nature Chemistry, a team led by Profs. Yang Xueming, Zhang Donghui, Dong Wenrui and Fu Bina from the Dalian Institute of Chemical Â鶹ÒùÔºics (DICP) of the Chinese Academy of Sciences has uncovered a surprising new pathway: syn-CH3CHOO's reaction with atmospheric water vapor is approximately 100 times faster than previously predicted by theoretical models.
Using advanced laser techniques, the researchers experimentally measured the reaction rate between syn-CH3CHOO and water vapor, and discovered the faster reaction time. To uncover the reason behind this acceleration, they constructed a high-accuracy full-dimensional (27D) potential energy surface using the fundamental invariant-neural network approach and performed full-dimensional dynamical calculations.
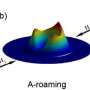
The researchers revealed a "roaming mechanism" driven by strong dipole–dipole interactions between the molecules. Instead of following a direct minimum energy path, the molecules "roam" near each other, leading to a much higher probability of reaction. Under typical atmospheric conditions, this water-based reaction pathway is just as important as the self-decomposition process that has been thought to dominate.
This finding suggests that the conventional view that unimolecular decomposition predominantly governs the removal of syn-CH3CHOO must be revised. By revising the understanding of key atmospheric processes, scientists may develop more accurate models of climate change and air quality. It also highlights the importance of combining high-precision experimental data with advanced full-dimensional simulations to accurately predict complex chemical reactions.
Beyond atmospheric science, the newly uncovered "roaming mechanism" could have wide-reaching implications, potentially affecting fields like combustion chemistry and astrochemistry, where long-range interactions play a major role in reaction dynamics.
More information: Yiqiang Liu et al, Reactivity of syn-CH3CHOO with H2O enhanced through a roaming mechanism in the entrance channel, Nature Chemistry (2025).
Journal information: Nature Chemistry
Provided by Chinese Academy of Sciences