This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers construct modularized catalytic system for transfer hydrogenation
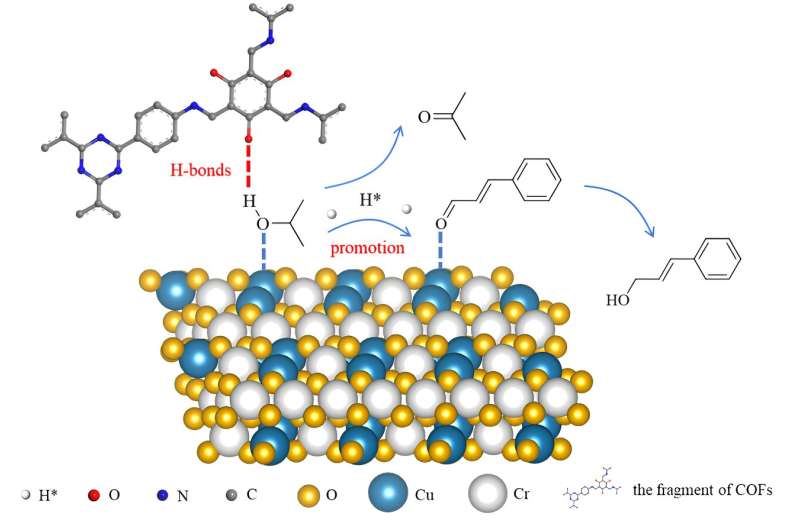
The precise catalytic conversion of chemical bonds is a paramount goal in catalysis. Enzymes, as efficient biocatalysts, are well known for their high catalytic activity, selectivity, and substrate specificity under mild reaction conditions, which can be attributed to the synergistic catalysis of multiple active sites.
Inspired by the catalytic mechanism of enzymes, the rational design of catalysts with multiple active sites to stabilize TS and accelerate the rate-determining step is a promising strategy for achieving high activity and selectivity.
However, integrating multiple active sites into a single catalyst without interference during the catalytic process remains an enormous challenge because it is difficult to combine different functional groups together at will, particularly incompatible groups.
Modularity is a method of decomposing complicated systems into various manageable submodules, each of which is independent of the other and works together in a certain way. Thus, it is highly desirable to construct a modularized catalytic system that mimics the synergistic catalysis of enzymes.
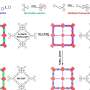
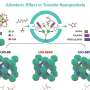
A research team led by Prof. Qihua Yang from Zhejiang Normal University, China, reported the construction of a modularized catalytic system for catalytic transfer hydrogenation (CTH) using covalent organic frameworks (COFs) and commercial Cu2Cr2O5 to simulate the function of amino acid groups and the active sites of enzymes, respectively.
The results were published in the .
In the CTH of different aldehydes with isopropyl alcohol, the modularized catalytic system with both COFs and Cu2Cr2O5 demonstrates enormously enhanced activity compared to Cu2Cr2O5. Mechanistic investigations and theoretical calculations suggest that COFs can interact with the hydroxyl group of isopropyl alcohol through hydrogen bonds, facilitating the dehydrogenation of isopropyl alcohol and promoting hydrogen atom transfer between isopropanol and aldehydes, thus improving catalytic activity.
In addition, the modularized catalytic system can be replaced by different submodules.
More information: Xin Liu et al, Construction of modularized catalytic system for transfer hydrogenation: Promotion effect of hydrogen bonds, Chinese Journal of Catalysis (2023).
Provided by Chinese Academy of Sciences