This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Detection of environmental PFAS by interrupted energy transfer
PFAS, a family of highly fluorinated substances, represent a danger for humans and the environment. Particularly problematic members of this family, such as perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) appear to cause organ damage and cancer, as well as disrupting the endocrine system.
The term per- and polyfluoroalkyl substances (PFAS) refers to a group of organic compounds in which most or all the hydrogen atoms bound to the carbon atoms have been replaced with fluorine atoms. They are used to provide water-, oil-, and dirt-resistance to a variety of products, such as nonstick pans, outdoor clothing, and packaging. They may also be found in fire-suppressing foam, paint, and car polish.
These compounds are highly useful—and highly dangerous when they find their way into the environment: they do not break down and thus become concentrated in plants, animals, and people.
Limits of 100 ng/l for individual specific PFAS substances and 500 ng/l for the total of all PFAS were set for drinking water in the EU. In Germany, water providers must begin testing drinking water for PFAS in 2026. The US Environmental Protection Agency has set stricter limits: for the most widespread PFAS (PFOS and PFOA), the upper limit is set at 4nm/l for each substance.
The usual method used to detect such trace amounts involves chromatography and mass spectrometry, is time-consuming and expensive, and requires complex equipment and experienced personnel. Timothy M. Swager and Alberto Concellón at the Massachusetts Institute of Technology (MIT) in Cambridge, U.S., have now introduced a technique for making a portable, inexpensive test that uses fluorescence measurements to easily and selectively detect PFAS in water samples.
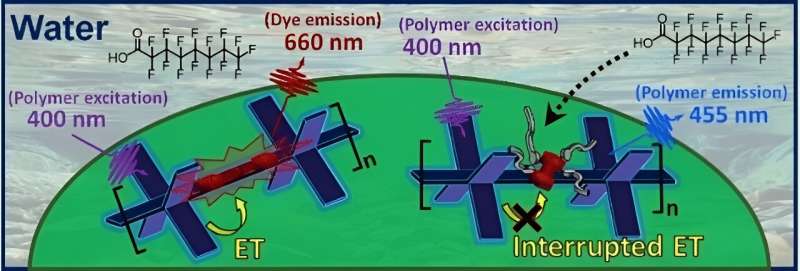
The test is based on a polymer—in the form of a thin film or nanoparticles—with fluorinated sidechains that have fluorinated dye molecules (squaraine derivatives) embedded in them. The special polymer backbone (poly-phenylene ethynylene) absorbs violet light and transfers the light energy to the dye by an electron exchange (Dexter mechanism).
The dye then fluoresces red. If PFAS are present in the sample, they enter the polymer and displace the dye molecules by a fraction of a nanometer. This is enough to stop the electron exchange and thus the energy transfer. The dye's red fluorescence is "switched off," while the blue fluorescence of the polymer is "switched on." The degree of fluorescence change is proportional to the concentration of PFAS.
This new technique, which has a detection limit in the µg/l range for PFOA and PFOS is suitable for on-site detection in highly contaminated regions. Detection of trace amounts of these contaminants in drinking water can be achieved with sufficient precision after pre-concentration of the samples by solid-phase extraction.
More information: Alberto Concellón et al, Detection of Per‐ and Polyfluoroalkyl Substances (PFAS) by Interrupted Energy Transfer, Angewandte Chemie International Edition (2023).
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Wiley