This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Opening a new frontier: PdMo intermetallic catalyst for promoting carbon dioxide utilization
Being the most abundant and persistent greenhouse gas emitted, carbon dioxide (CO2) is the key driver of climate change. To address the pressing problems associated with climate change and fossil fuel depletion, scientists are looking for viable solutions that can minimize the amount of CO2 released into the atmosphere.
One attractive solution to this problem is to convert atmospheric CO2 into more useful compounds. Towards this end, methanol—a raw material, fuel additive, and energy carrier—is widely being explored as a promising conversion option for CO2.
While various catalysts are currently used for CO2 conversion reactions, most of them were designed and investigated for use in high-temperature and -pressure conditions. This is a serious limitation for multiple reasons.
First, maintaining such conditions requires energy and expensive containment systems. Second, the CO2 hydrogenation reaction is exothermic, and thus proceeds more favorably at lower temperatures. Third, high temperatures can sometimes compromise the stability of catalysts, resulting in their reduced lifespan. Finally, the conversion efficiency of existing heterogenous catalysts is extremely low for catalyzing such conversion reactions.
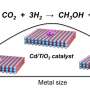
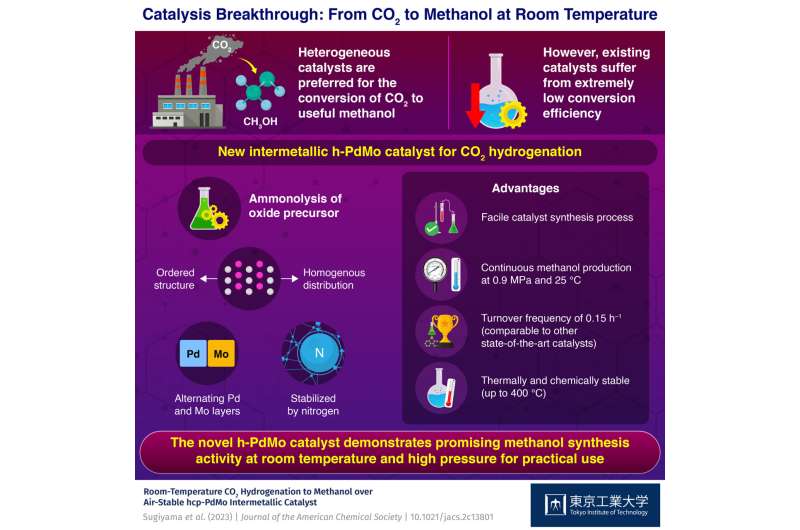
Against this backdrop, a team of researchers led by Professor Hideo Hosono from Tokyo Institute of Technology, Japan, set out to develop a better catalyst for CO2 hydrogenation. In their study published in the Journal of the American Chemical Society, they report the development of a novel intermetallic catalyst synthesized via a simple ammonolysis process by combining palladium (Pd) and molybdenum (Mo).
To synthesize this catalyst, the researchers employed a simple approach based on ammonolysis of an oxide precursor. Put simply, ammonolysis can be used to combine metals by mixing precursors, such as oxides or nitrates, with ammonia gas at high temperatures. Ammonia reacts with the precursors to form intermediate complexes called metal amides, which then decompose to form the desired intermetallic compound.
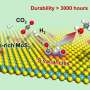
Using various analytical techniques, the team determined the crystal structure of the "h-PdMo" catalyst and probed its chemical and thermal stability. Notably, they found that h-PdMo was stable at temperatures up to 400 °C and did not decompose in air. "This kind of robustness is very important when considering the practicality of a catalyst," remarks Prof. Hosono.
Next, the researchers evaluated the performance of h-PdMo for CO2 hydrogenation under different conditions. At a temperature of 100 °C, the catalyst was capable of continuous methanol production without any significant sign of degradation, for over 100 hours. Moreover, at room temperature (25 °C) and under relatively low pressure, the performance of h-PdMo was remarkable.
Explaining the findings further, Prof. Hosono says, "At a pressure of 0.9 MPa, our catalyst achieved a conversion efficiency comparable to or even higher than that of state-of-the-art heterogeneous catalysts, that demonstrate similar turn over efficiency under higher-pressure conditions in the range of 4 to 5 MPa."
In summary, the researchers developed a very active and stable catalyst for CO2 hydrogenation at room temperature that can be synthesized via a simple process. Prof. Hosono concludes by saying, "Our discovery provides a frontier for catalyst development, not only for low-temperature methanol synthesis and CO2 conversion reactions, but also for other reactions catalyzed by Pd."
More information: Hironobu Sugiyama et al, Room-Temperature CO2 Hydrogenation to Methanol over Air-Stable hcp-PdMo Intermetallic Catalyst, Journal of the American Chemical Society (2023).
Journal information: Journal of the American Chemical Society
Provided by Tokyo Institute of Technology