Examining the contribution of water molecules to the hydrogen evolution reaction

Coupling renewable energy to produce hydrogen by the electrolysis of water is an effective way to develop new energy utilization and solve the energy crisis. It is important to fully understand the cathodic hydrogen evolution reaction (HER) mechanism.
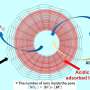
Compared with the fast kinetics under acidic conditions, the kinetics under alkaline conditions are two orders of magnitude slower, indicating that the reaction mechanism becomes more complicated than that under acidic conditions. Therefore, an in-depth understanding based on the descriptor of the HER mechanism under alkaline conditions is of practical significance for improving the HER activity.
Researchers at Chongqing University stated that "water molecules act as solvents in catalyzing HER in acid, while water molecules play dual roles in catalyzing alkaline HER, that is, reactant and solvent." Based on their research and knowledge in the field of HER, the researchers focused on the contribution of water molecules to HER, summarized the development of the activity descriptor of HER in the past decade, and provided their understanding of how the water molecules affect the kinetics and dynamics of HER.
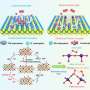
For instance, the commonly used activity descriptor, hydrogen binding energy, can denote the alkaline HER activity after being corrected by water adsorption energy. Water dissociation involved the hydroxide binding, and the OH- and cation adsorption was recognized as descriptors for HER. Moreover, the interfacial water is highlighted as a crucial factor affecting pH-dependent HER activity, and it can be deliberately enriched and reoriented to improve HER kinetics dramatically.
The review paper is published in Science China Chemistry. The work is led by the head of the New Energy Material Chemistry and Chemical Engineering Group, Zidong Wei. Chao Cheng and Mingming Deng are the first authors. The group has spent years researching the underlying mechanism of electrocatalysis, designing novel electrocatalysts with high performance, thus improving the conversion efficiency between matter and energy in the new energy field, including water electrolysis, fuel cells, etc.
Water molecule as the reactant in HER
The researchers say that "water molecules are the few molecules that can enter the depth of the electrode/electrolyte interface because of a strong interaction between the charged electrode and water dipole," and "in neutral and alkaline solutions, water acts as a reactant to supply the source of hydrogen evolution. The continuous HER will be suppressed if water is weakly adsorbed on the catalyst surface and not provided timely."
The role of interfacial water in catalyzing HER
"Interfacial water configuration and dynamic process are of high importance in determining the HER performance of catalysts," said Zidong Wei. "On the basis of our previous studies, we concluded that the electric field at the electrode/solution interface can regulate the enrichment, reorientation, and activation dissociation of interfacial water, thereby promoting the coupling of the elementary steps of HER and improving its reaction dynamics in the high polarization region, and this coupling mechanism is also universal."
More information: Chao Cheng et al, The contribution of water molecules to the hydrogen evolution reaction, Science China Chemistry (2022).
Provided by Science China Press