This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Atomic pair catalyst converts methane to acetic acid with high efficiency

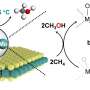
In a study in Nature Communications, a research group led by Prof. Deng Dehui, Assoc. Prof. Cui Xiaoju, and Prof. Yu Liang from the Dalian Institute of Chemical Â鶹ÒùÔºics (DICP) of the Chinese Academy of Sciences has achieved highly efficient photo-driven carbonylation of methane (CH4) with carbonic oxide (CO) and oxygen (O2) to acetic acid (CH3COOH) using a nano-heterostructure catalyst.
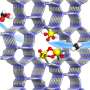
This catalyst features Rh-Zn atomic-pair dual sites confined within a MoS2 lattice, integrated with TiO2 nanoparticles. This innovative catalyst enables a CH3COOH productivity of 152 μmol gcat.-1 h-1, and a turnover frequency of 62 h-1 with a high selectivity of 96.5%.
The direct conversion of CH4 to high value multi-carbon (C2+) oxygenates, such as CH3COOH, under mild conditions presents a promising pathway for upgrading natural gas to transportable liquid chemicals.
The oxidative carbonylation of CH4 with CO and O2 to CH3COOH under mild conditions is an attractive and environmentally friendly route for CH4 utilization. However, this process involves complex reactions, including the activation of O2, efficient CH4 activation, and controllable C–C coupling. It's therefore a major challenge to achieve CH4, CO, and O2 to CH3COOH with both high catalytic activity and selectivity for mild CH4 conversion.
In this study, the researchers showed that the active OH species, generated from O2 photoreduction at the Zn site by proton-coupled electron transfer, promote CH4 dissociation to CH3 species. These CH3 species then easily couple with adsorbed CO at the adjacent Rh site, leading to highly selective CH3COOH formation.
Additionally, the dual Rh–Zn atomic-pair sites provide separate catalytic sites for C–H activation and C–C coupling, creating a synergistic effect that overcomes the typical trade-off between activity and selectivity in CH4 carbonylation.
"Our study opens up a new horizon for the design of efficient catalysts and provides a new pathway for photo-driven CH4 carbonylation to CH3COOH," said Prof. Deng.
More information: Yanan Li et al, MoS2-confined Rh-Zn atomic pair boosts photo-driven methane carbonylation to acetic acid, Nature Communications (2025).
Journal information: Nature Communications
Provided by Chinese Academy of Sciences