This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists explore whether smaller chromosomes lead to more segregation errors during egg cell division

Three RIKEN researchers have shed light on the cellular mechanisms underlying chromosomal abnormalities that can cause miscarriages and congenital disorders such as Down syndrome.
These abnormalities often arise when sex cells in humans—egg cells (oocytes) in women and sperm cells in men—don't divide properly and transfer too many or too few chromosomes to an embryo. Roughly a third of miscarriages occur for this reason.
Mistakes that cause this typically happen when chromosomes separate during cell division, so scientists call them chromosome segregation errors. But how these errors occur is still a mystery.
The likelihood of these errors increases with age, particularly in women. Tomoya Kitajima of the RIKEN Center for Biosystems Dynamics Research is interested in chromosome segregation errors associated with aging because Japan and other countries are grappling with declining birth rates.
"We should be able to have babies when we desire," he says. "But we lack the technology to overcome the problems of chromosome segregation errors."
In 2015, Kitajima's team found that the main reason for chromosome segregation errors is the tendency of some chromosomes to split up before the cell is ready for division.
Now, the trio has explored in mice whether smaller chromosomes are more likely to separate prematurely and cause segregation errors. The work is in the journal Science.
"We knew that smaller chromosomes were more prone to errors," notes Kitajima.
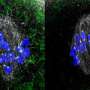
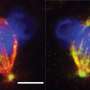
The researchers isolated oocytes from the ovaries of female mice and injected them with special RNA molecules, which ensured that fluorescent proteins are delivered to target chromosomes in the cell.
When observed under powerful microscopes, the chromosomes glowed due to the fluorescent proteins, allowing the team to capture footage of chromosomes prancing around the cell.
Statistical analyses of the videos revealed that smaller chromosomes tended to huddle near the core of the spindle—a fibrous structure inside an oocyte that facilitates chromosome division—whereas the larger chromosomes enclosed them.
The spindle has push-and-pull forces that help line up chromosomes for division. These forces are particularly strong in the inner area of the spindle and can split up chromosomes too soon.
The glue that binds chromosomes tends to weaken in aging oocytes, rendering the smallest chromosomes particularly vulnerable in these cells.
This formation—smaller chromosomes surrounded by larger counterparts—is also seen in other cells when they are not dividing. But the researchers didn't expect to stumble upon it during this study. "It came as quite a surprise," recalls Kitajima.
The findings might help boost reproductive technologies in the future, potentially enabling healthy pregnancies in older women. "If we can manipulate such a spatial arrangement, we may be able to prevent errors," says Kitajima.
More information: Osamu Takenouchi et al, Live chromosome identifying and tracking reveals size-based spatial pathway of meiotic errors in oocytes, Science (2024).
Journal information: Science
Provided by RIKEN