This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers create a glass that sifts carbon dioxide
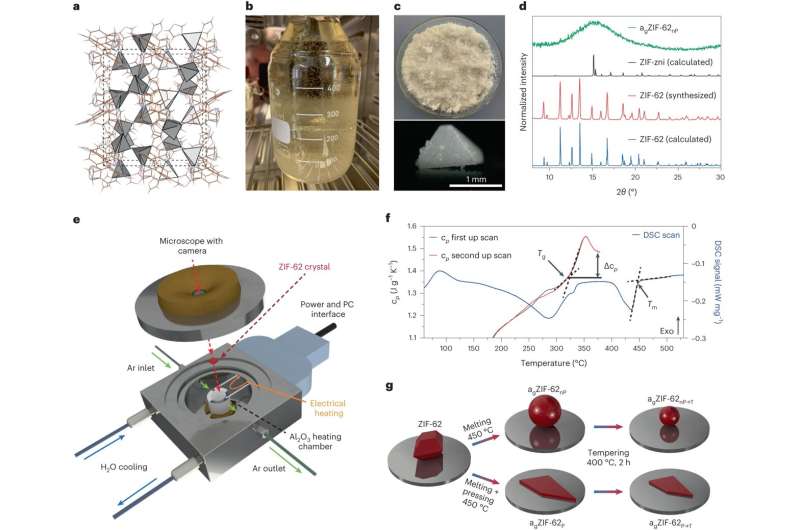
Separating carbon dioxide molecules from gas mixtures requires materials with extremely fine pores. Researchers from Friedrich Schiller University Jena, in cooperation with the University of Leipzig and the University of Vienna, have now found a novel way to do this.
They transformed crystalline metal-organic framework compounds into glass. In doing so, they managed to reduce the pore size of the material to the point where it becomes impermeable to certain gas molecules. They have reported their findings in the journal .
Compressed metal-organic frameworks
"Actually, these glass-like materials were previously considered non-porous," explains Dr. Alexander Knebel from the Otto Schott Institute of the University of Jena, who led this work. "The starting material, i.e., the crystalline framework compounds have very clearly defined pores and also a large internal surface area. Hence, they are also researched as materials for storing or separating gases. However, this defined structure is lost during melting and compression. And we took advantage of that."
"Metal-organic framework compounds consist of metal ions linked together by rigid organic molecules," says the leader of the junior research group. "In the spaces of these three-dimensional, regular grids, gas molecules can move easily. During the glass processing, we compressed the material. Put simply, we were able to squeeze the pores down to the desired size."
Ordered disorder
Even though the overall structure of the crystal disappears during melting—parts of the crystal retain their structure. "In technical terms, this means: during the transition from crystal to glass, the long-range order of the material is lost, but the short-range order is preserved," explains Knebel.
Oksana Smirnova, a doctoral student at the University of Jena and the lead author of the work, adds, "When we now melt and compress this material, the porous interstices also change." As a result, channels with constrictions—or even dead ends—are created, and consequently, some gases simply no longer fit through.
In this way, the group achieved pore diameters of 0.27 to 0.32 nanometers in the material, with an accuracy of one-hundredth of a nanometer. "For illustration: This is about 10,000 times thinner than a human hair and 100 times thinner than a DNA double helix. With this pore size, we were able to separate, for example, carbon dioxide from ethane," explains Knebel. "Our breakthrough in the field is probably the high quality of the glasses and the precise adjustability of the pore channels. And our glasses are also several centimeters in size."
"One goal of this work is to develop a glass membrane for environmental applications. Because separating carbon dioxide from gases is undoubtedly one of the great technological challenges of our time," says Knebel. "That's why I am also grateful ... for the outstanding commitment of my doctoral student Oksana Smirnova, who contributed significantly to the success of this work."
More information: Oksana Smirnova et al, Precise control over gas-transporting channels in zeolitic imidazolate framework glasses, Nature Materials (2023).
Journal information: Nature Materials
Provided by Friedrich Schiller University of Jena