This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Researchers reveal drug resistance mechanism of pathogen Staphylococcus aureus
Recently, a research team led by Prof. Sun Baolin from the University of Science and Technology of China (USTC) revealed the mechanism of transcriptional regulation via S-nitrosylation for vancomycin resistance in Staphylococcus aureus. Their work was published in Nature Communications.
Staphylococcus aureus (S. aureus) is a major human pathogen, and infections caused by methicillin-resistant S. aureus (MRSA) are posing serious threats to public health. Vancomycin is considered to be a last-resort drug in the treatment of severe MRSA infections, but the frequent emergence of vancomycin-intermediate S. aureus (VISA) brings a great challenge.
Nitric oxide (NO), a signaling molecule, was first discovered to be endogenously generated by nitric oxide synthase (NOS) in eukaryotes and is involved in regulating various physiological and immune functions. Previous research has reported that NO can mediate the S-nitrosylation modifications of cysteine residue, consequently affecting the activity and functions of protein. Researchers have verified that NOS exist in S. aureus and are involved in regulating the vancomycin resistance, but the molecular mechanism remains unclear.
To reveal the mechanism, researchers first constructed an NOS mutant strain and then added NOS inhibitor exogenously in a clinically isolated VISA strain XN108. Then, proteomic analysis identified the target protein and corresponding sites that can be modified by NO-mediated S-nitrosylation in S. aureus.
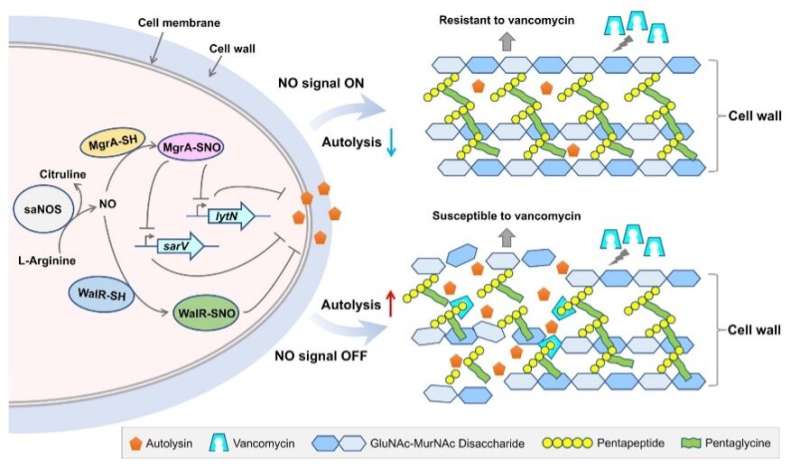
MgrA, a transcription regulator involved in antibiotics resistance, was found to be S-nitrosylated at cysteine residue Cys12. Researchers generated the mgrAC12S mutant strain by replacing the Cys12 with a serine, which can't be S-nitrosylated. The mgrAC12S mutant strain showed a significant decrease in vancomycin resistance and cell wall thickness as well as an increase in autolytic activity.
The team used methods like fluorescent quantitative PCR, gel-shift experiment and chromatin immunoprecipitation assay to reveal the roles of S. aureus NOS and the endogenously generated NO in promoting vancomycin resistance. NO generated by NOS mediates the S-nitrosylation of MgrA, which negatively regulated autolysis in S. aureus, causing an increase in cell wall thickness, thereby promoting the vancomycin resistance.
Such NO-mediated regulation mechanism was further verified in another transcription regulator WalR that can be modified by S-nitrosylation, indicating that this mechanism could be universal in bacteria.
This study is expected to provide new ideas and strategies for the clinical treatment of VISA and other bacterial pathogens infections.
More information: Xueqin Shu et al, Transcription tuned by S-nitrosylation underlies a mechanism for Staphylococcus aureus to circumvent vancomycin killing, Nature Communications (2023).
Journal information: Nature Communications
Provided by University of Science and Technology of China