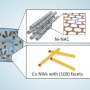
Mechanism of the anode-free Zn electrodeposition. a Schematic diagrams of Zn electrodeposition on Zn and Sb/Sb2Zn3-HI@Cu substrates (HI represents heterostructured interface). b Nucleation barriers of the Zn electrodeposition on Zn, Cu and Sb@Cu substrates, where the charge current density is 3 mA cm−2 in 2 M ZnBr2. c XRD patterns of the Zn electrodeposition and stripping on Sb@Cu at different capacities. d XPS spectra of Zn 2p of Zn plating on Sb@Cu substrate with different capacities. e HRTEM images of the Sb2Zn3-HI@Cu anode. f EDS-mapping of the Zn and Sb in the Sb2Zn3-HI@Cu anode. The sample for HRTEM and EDS-mapping measurements was prepared by plating 0.2 mAh Zn on Sb@Cu substrate. The constant current electrodeposition shown in Fig. 1b was carried out at room temperature (25 °C). Credit: Nature Communications (2023). DOI: 10.1038/s41467-022-35630-6
Recently, a research team led by Prof. Zeng Jie from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) constructed a stable single-site copper coordination polymer that largely improved the efficiency of ethylene production by single-atom catalytic electroreduction of carbon dioxide. This work was published in Nature Communications.
Single-site catalysts have attracted great attention due to their clear structure, high utilization ratio and high selectivity. It is commonly agreed that copper particles catalyze the reduction of CO2 to polycarbonate products, while copper monoatomic catalysts catalyze the reduction of CO2 to methane.
It has been reported that some monoatomic catalysts are prone to agglomerate to form copper particles during the electrochemical process, which yields polycarbonate products. However, it remains unclear how to design stable single-site catalysts for the synthesis of CO2 electroreduction processes while efficiently catalyzing carbon-carbon coupling.
In this study, the researchers constructed a stable single-site copper coordination polymer (Cu(OH)BTA) to realize the efficient carbon-carbon coupling process by electroreduction of CO2.
Structural analysis showed that the coordination polymer featured near-neighboring periodic copper sites, and at a suitable copper-copper distance, carbon-carbon coupling occurred without destabilizing the catalyst structure due to the adsorption of intermediates, achieving efficient synthesis of multi-carbon products on a stable single-site catalyst.
The electroreduction of carbon dioxide in the flow cell showed that the Faradaic efficiency of the multi-carbon product was greatly improved compared to that of copper metal. Membrane electrode reaction cell performance evaluation further demonstrated the excellent CO2 electrocatalytic performance of Cu(OH)BTA.
A series of characterizations such as in situ X-ray absorption spectroscopy and in situ Raman demonstrated that the mild local environment of this coordination polymer maintained structural stability during electrocatalytic CO2 reduction. The near-neighboring copper sites provided the active center with a suitable distance for the carbon-carbon coupling process and promoted the formation of the key adsorption intermediate (*OCCHO).
The study provides new ideas for the design of molecularly stable catalysts for activating and converting CO2.
More information: Xinhua Zheng et al, Constructing robust heterostructured interface for anode-free zinc batteries with ultrahigh capacities, Nature Communications (2023).
Journal information: Nature Communications
Provided by Chinese Academy of Sciences