Exploiting molecular vibrations to synthesize conducting polymers
Conjugated polymers are organic macromolecules that are characterized by a backbone chain of alternating double and single bonds. Their overlapping p-orbitals create a cloud of delocalised π-electrons, which can result in useful optical and electronic properties. The design of π-conjugated polymers is highly desirable for technological applications such as tailor-made components for nanoelectronics.
Conjugated polymers synthesized by wet chemistry present defects that are incompatible with the requirements of atomic-precise electronics. The topology of the π-electron network is crucial, since it determines the ground state electronic structure of such materials. It is therefore interesting to design protocols to produce low-bandgap π-conjugated polymers. On-surface chemistry is a promising procedure that allows the engineering of such macromolecules with control over the synthesis and structural characterization at the atomic scale by means of scanning probe microscopy.
Researchers from the Czech Republic and Spain have joined efforts to design chemically robust and low-bandgap polymers. Their proposed unconventional strategy exploits the relation between π- conjugation and specific vibrational modes in order to increase the attempt frequency of a chemical reaction, thus introducing the importance of vibrational modes in chemical design on surfaces.
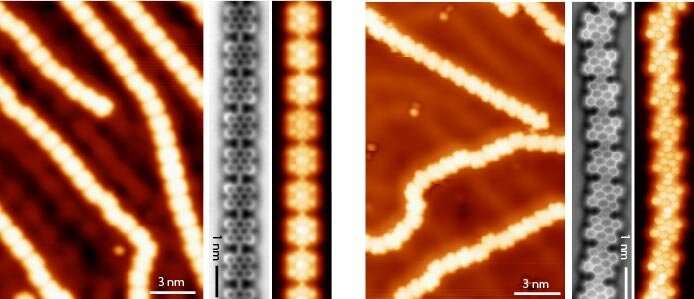
They designed a one-atom-thick polymer on Au(111) based on bisanthene monomers linked by cumulene bridges that exhibit specific vibrational modes (see figure, left panel). In a second step, upon further annealing, such vibrational modes steer the reaction between adjacent bisanthene moieties, which gives rise to a long pentalene-bridged polymer featuring a low bandgap (figure, right panel). The resonance and electronic properties of the products were characterized in detail by scanning tunneling microscopy (STM) and non-contact atomic force microscopy (nc- AFM) supported by density functional theory (DFT) study of the on-surface reactions.
"Our results introduce the relevance of tailoring π-conjugation resonance form to steer vibrational modes on surfaces with the aim of promoting otherwise precluded chemical reaction pathways," says Prof. David Écija.
"The study indicates that in addition to the transition state, the internal vibrational modes of the reactant may play an important role in reaction mechanisms," says Prof. Pavel JelÃnek.
"We envision our results will open pathways to design highly demanded conjugated nanomaterials, while showing strategies to incorporate non-benzenoid moieties in polymeric science," says Prof. Nazario MartÃn.
More information: B. de la Torre et al. Tailoring π-conjugation and vibrational modes to steer on-surface synthesis of pentalene-bridged ladder polymers. Nature Communications. 11, 4567 (2020).
Journal information: Nature Communications
Provided by IMDEA Nanociencia



















