Chemist develops technique to improve solar cells
A RUDN University chemist has discovered four new stable compounds that can be obtained in the reaction of iodine with methylammonium iodide—the use of these substances will allow the production of perovskite solar panels without toxic reagents and prevent by-products during manufacturing. The article is published in The Journal of Â鶹ÒùÔºical Chemistry Letters.
Lead-based hybrid perovskites are used in modern solar cells as a light-absorbing layer. But they are unstable to moisture, and existing technologies require the use of solutions and toxic solvents. This complicates the technology and makes it potentially dangerous.
The solution to the problem could be solvent-free methods, that is, the use of melts rather than solutions—for example, applying a polyiodide melt to a thin film of metallic lead. However, there are few reliable studies of polyiodide chemistry. Researchers studied the properties of methylammonium (CH3NH3) and iodine compounds to find variants of compounds suitable for use in the production of perovskite solar cells.
Compounds of the system methylammonium iodide (MA) and iodine melt at room temperature and form ionic liquids—melts that are composed exclusively of ions. These precursor liquids can be evenly applied to large surfaces, bringing the industrial production of modular solar cells based on hybrid perovskites for the commercial market.
Liquids based on polyiodides melt at room temperature only in the presence of large organic cations in the composition. RUDN University chemist Victor Khrustalev explained this difference by the fact that the methylammonium cation has a large dipole moment and is capable of forming a large number of hydrogen bonds. At small cation sizes, this leads to increased entropy during melting, which lowers the melting temperature.
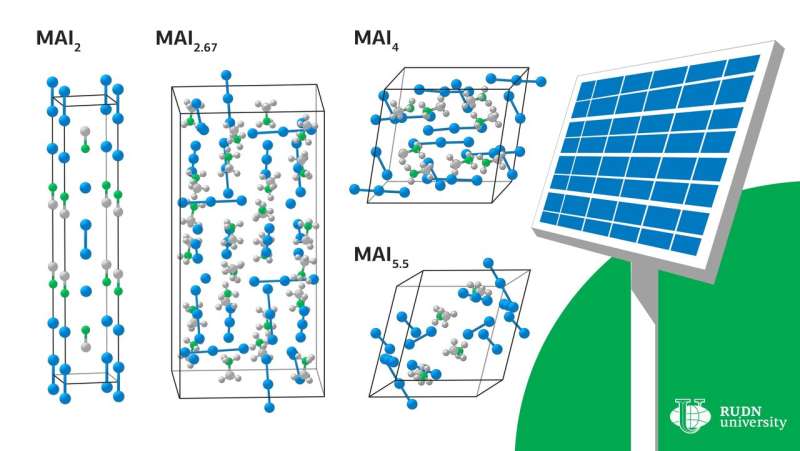
Under certain conditions, crystals of various compositions begin to evolve out of the liquid—MAI2, MAI2.67, MAI4 и MAI5.5. To determine the conditions under which there is a melt, scientists have carried out a theoretical calculation of the enthalpy and entropy of the formation of these crystals. For all compounds except MAI2, the reaction of obtaining a compound with a lower iodine content depended solely on the entropy contribution. Сhemists explained that the increase in enthalpy during the transition to compounds with a higher iodine content is due to the weakening of the interaction of cations with anions due to the distribution of a small negative charge on a large polyiodide anion. A similar increase in entropy is due to the complexity of polyanions and the weakening of the bonds between them.
These thermodynamic data allowed us to define and generalize the experimental values of the boundaries in which the melt can exist.
Chemists have also found that such effects occur in similar compounds with the formamidine cation (FA+ = HC(NH2)2) and polybromides. Moreover, the mixed composition (MABr3)0.15(FAI3)0.85 demonstrates ionic liquid properties from -40 to 80 °C. Such a low melting point of the precursor is favorable for obtaining thin films of mixed hybrid perovskites that demonstrate maximum light-absorbing properties.
More information: Andrey A. Petrov et al. Methylammonium Polyiodides: Remarkable Phase Diversity of the Simplest and Low-Melting Alkylammonium Polyiodide System, The Journal of Â鶹ÒùÔºical Chemistry Letters (2019).
Journal information: Journal of Â鶹ÒùÔºical Chemistry Letters
Provided by RUDN University