July 12, 2019 feature
Creating two-dimensional layered Zintl phase by dimensional manipulation of the crystal structure
![Creation of 2D layered ZnSb. (A) Schematic illustration of the dimensional manipulation of a crystal structure from 3D-ZnSb to 2D-ZnSb via Li alloying and etching processes. The Li alloying into 3D-ZnSb was conducted by thermal and electrochemical reactions (ERs). The selective etching of Li ions was conducted by reacting with polar solvent solution reaction (SR). A reversible process of alloying and etching occurs in the mean of electrochemical reaction (ER). (B) XRD patterns of 3D-ZnSb and 2D-LiZnSb. The 2D-LiZnSb polycrystal and single crystal were synthesized by using the synthesized 3D-ZnSb as a precursor. All patterns are well matched with the simulated patterns of corresponding compounds. a.u., arbitrary units. (C) XRD patterns of 2D-ZnSb crystals obtained by solution reaction and electrochemical reaction processes. For the solution reaction process, water-based solutions [DI water and dimethyl sulfoxide (DMSO) with 1 volume % of DI water, and hexamethyl phosphoric triamide (HMPA) with 1 volume % of DI water] were used. For the electrochemical reaction process, 1 M LiPF6 dissolved in 1:1 mixture of ethylene carbonate and diethyl carbonate solution was used as an electrolyte. The interlayer distances were calculated from the angle of highest intensity. (D to I) Scanning electron microscopy (D to F) and optical images (G to I) of 2D-LiZnSb and 2D-ZnSb created by the solution reaction and electrochemical reaction processes. The flakes of 2D-ZnSb were exfoliated by mechanical cleaving using 3M tape. (J to L) X-ray photoelectron spectroscopy (XPS) spectra of Li 1s (J), Zn 2p (K), and Sb 3d (L) for 3D-ZnSb, 2D-LiZnSb, and 2D-ZnSb, respectively. The Li 1s peak (54.6 eV) of 2D-LiZnSb indicates the Li1+ state. While the binding energies of the Zn 2p3/2 (1019.8 eV) and Sb 3d5/2 (525.8 eV) are significantly lower than the Zn 2p3/2 (1021.5 eV) and Sb 3d5/2 (527.6 eV) in 3D-ZnSb, the binding energies of Zn 2p3/2 (1022.1 eV) and Sb 3d5/2 (528.2 eV) of 2D-ZnSb are slightly higher than those of 3D-ZnSb. Credit: Science Advances, doi: 10.1126/sciadv.aax0390 Creating two-dimensional layered Zintl phase by dimensional manipulation of the crystal structure](https://scx1.b-cdn.net/csz/news/800a/2019/creatingtwod.jpg)
The discovery of new families of two-dimensional (2-D) layered materials beyond graphene has always attracted great attention, but it remains challenging to artificially recreate the honeycomb atomic lattice structure with multi-components such as hexagonal boron nitride in the lab. In a new study now published on Science Advances, Junseong Song and colleagues at the departments of Energy Science, Nanostructure Â鶹ÒùÔºics, Environmental Science and Materials Science in the Republic of Korea developed an unprecedented structure of the Zintl phase.
They constructed the material by staking honeycomb ZnSb layers and via the dimensional manipulation of a crystal structure from the 3-D-ZnSb state. The materials scientists combined structural analysis with theoretical calculations to form a stable and robust layered structure of 2-D-ZnSb. This phenomenon of bi-dimensional polymorphism was not previously observed at ambient pressure in Zintl families. Therefore, the new work provides a rational design strategy to search and create new 2-D layered materials in various compounds. The new results will allow the unlimited expansion of 2-D libraries and their corresponding physical properties.
The advent of Dirac physics of graphene triggered an explosive interest in research on two-dimensional (2-D) materials with varied applications in , , and chemistry to . At present, 2-D research is primarily focused on a containing a single or exfoliated from their mother compounds, in contrast to . This can restrict the method of 2-D materials development to two approaches of and chemical vapor deposition. It is therefore highly desirable to expand on 2-D materials research to artificially create a new 2-D material with a new synthetic approach and form a variety of material groups.
In new materials discovery, transformation of a crystal structure is a widely recognized key factor. Where the temperature-pressure and electrostatic-doping induced structural phase transitions are core to or to properties. For instance, most transition exhibit polymorphic phase transition to access inherently diverse properties including superconducting and topological states. The transition has led to promising applications including , photonic memory devices and .
![Crystal structure of 2D layered ZnSb. (A and B) Atomic resolution STEM-HAADF (high-angle annular dark-field) images of 2D-LiZnSb along the [110] (A) and [001] (B) zone axes, respectively. (C) Atomic resolution STEM-EDS elemental mapping for 2D-LiZnSb along the [110] (top) and [001] (bottom) zone axes. (D and E) Atomic resolution STEM-HAADF images of 2D-ZnSb along the [110] (D) and [211] (E) zone axes. The determined crystal structure of 2D-ZnSb. The atomic distances of 2D-ZnSb are compared with those of 3D-ZnSb and 2D-LiZnSb. From the observation at [211] zone axis of 2D-ZnSb, the honeycomb lattice is slightly tilted. For the detection of lithium, the STEM–EELS (electron energy-loss spectroscopy) technique was used, showing the clear existence and absence of lithium in 2D-LiZnSb and 2D-ZnSb. (G) Cohesive energy (ΔEcoh) calculation of predictable 2D-ZnSb structures. Structure I that is determined from the STEM observations exhibits the lowest energy compared with other candidates, showing excellent agreement between experiments and calculations. Credit: Science Advances, doi: 10.1126/sciadv.aax0390 Creating two-dimensional layered Zintl phase by dimensional manipulation of the crystal structure](https://scx1.b-cdn.net/csz/news/800a/2019/1-creatingtwod.jpg)
These polymorphic transitions only occurred between different layered structures in the same two dimensions and remain to be realized between different dimensions of a crystal structure at ambient pressure. To reach ultimate crystal engineering and alter the structural dimension of multicomponent compounds is a promising next frontier in materials science beyond the .
In the present work, Song et al. established via the discovery of 2-D layered structures in Zintl phases . Due to the sp2 hybrid orbital bonding of honeycomb-structured 2-D atomic crystals such as graphene and hexagonal boron nitride, the scientists expected the 3-D structured Zintl phases (with sp3 hybrid orbital bonding) to transform to sp2 honeycomb structured 2-D layered materials, as well, via electron transfer. As a proof-of-concept, Song et al. selected a 3-D ZnSb (3-D-ZnSb) Zintl phase and created the unprecedented, 2-D layered structure of ZnSb (2-D-ZnSb).
In the new method, Song et al. first synthesized layered AZnSb (2-D-AZnSb) ; where A referred to an alkali metal such as Na, Li and K. The materials contained a layered structure of ZnSb by transforming 3-D-ZnSb via A alloying, although the phases could be . Song et al. performed selective etching of A ions to create the 2-D-ZnSb in two different processes, including (1) chemical reaction in deionized water-incorporated solutions, and (2) electrochemical ion etching reaction in alkali-based electrolyte.
For example, they synthesized the polycrystalline and single crystalline 2-D-LiZnSb intermediate substrate by first alloying Li into polycrystalline 3-D-ZnSb, followed by Li ion etching to form a 2-D-ZnSb crystal. The scientists easily cleaned the Li-etched 2-D-ZnSb crystals using adhesive tape exfoliation as mechanical cleaving to exhibit .
To understand the effect of the manufacturing process, they examined the role of Li alloying and etching on structural transformations using X-ray photoelectron spectroscopy (XPS) measurements to reveal the difference between the 2-D and 3-D crystals. To further validate their findings, Song et al. used X-ray diffraction spectroscopy (XRD) patterns, transmission electron microscopy (TEM) observations and (STEM) combined with (EDS) elemental mapping to confirm the atomic structure of 2-D-ZnSb.
Based on the results, the scientists interpreted the stretchable interlayer distances between both Zn-Zn and Sb-Sb atoms as weak interlayer bonds and verified that the 2-D-ZnSb could be exfoliated as a layered material. The newly evolved layered structure of 2-D-ZnSb in the present work, completed the first discovery of bidimensional polymorphism in Zintl phases at ambient pressure.
![2D layered behavior of 2D-ZnSb. (A) [100] view of 3D-ZnSb. (B) [100] view of 2D-ZnSb. (C) Cohesive energy (ΔEcoh) calculation of 3D-ZnSb, 2D-ZnSb. From the cohesive energy calculation, 3D-ZnSb is more stable but the cohesive energy of 2D-ZnSb is reasonably large enough, indicating that the 2D-ZnSb exists as a stable material. (D) Li alloying energy (ΔELi alloying) calculation of 3D-ZnSb and 2D-ZnSb indicating reaction Li alloying process in 2D-ZnSb and 3D-ZnSb are energetic favor. Compare two ΔELi alloying, Li ions alloying into 2D-ZnSb is favorable than 3D-ZnSb. (E) Interlayer binding energy (Einter) of 3D-ZnSb and 2D-ZnSb. Large difference of Einter between 3D-ZnSb and 2DZnSb indicates the characteristics of 2D layered materials for 2D-ZnSb. (F) Exfoliation energy (Eexf) calculation of 2D-ZnSb and other 2D materials. Eexf of 2D-ZnSb is rather higher than those of conventional van der Waals (vdW) bonded 2D materials such as graphene and h-BN, indicating that the 2D-ZnSb is not a vdW-type layered material. However, the Eexf of 2D-ZnSb is lower than that of antimonene, which can be exfoliated or grown into monolayer indicating that free-standing monolayer or few layers of 2D-ZnSb can be possible as conventional 2D vdW layered materials. Credit: Science Advances, doi: 10.1126/sciadv.aax0390. Creating two-dimensional layered Zintl phase by dimensional manipulation of the crystal structure](https://scx1.b-cdn.net/csz/news/800a/2019/3-creatingtwod.jpg)
Accordingly, Song et al. manipulated the sp3-hybridized bonding state in 3-D-ZnSb into the sp2 state in 2-D-ZnSb honeycomb lattice. Previous studies on polymorphic transitions between 3-D and 2-D structures in Zintl phases were only . The present results on bidimensional polymorphism between 3-D-ZnSb and 2-D-ZnSb emphasized the potential and broad availability of such an electron transfer to transform the crystal structure.
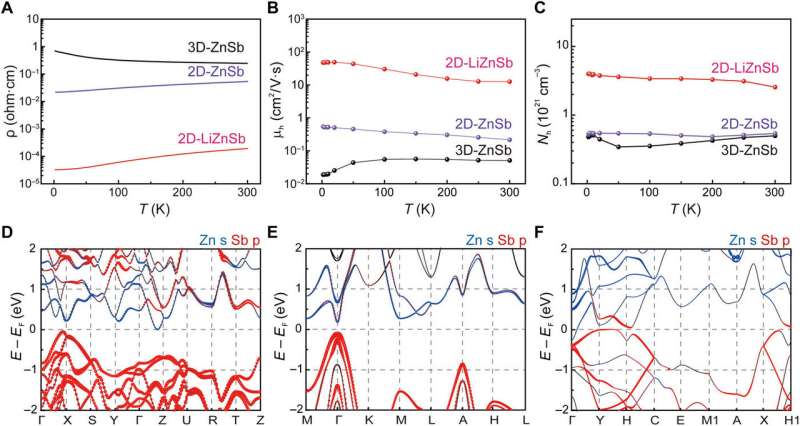
Song et al. next investigated electrical transport properties of bidimensional ZnSb polymorphs and 2-D-LiZnSb crystals alongside first principles calculations of their electronic energy band structure. In contrast to the semiconducting nature of 3-D-ZnSb, both 2-D-LiZnSb and 2-D-ZnSb showed metallic conduction behavior. When they decreased the temperature, the electrical mobilities of both 2-D-LiZnSb and 2-D-ZnSb increased to a value higher than that of 3-D-ZnSb. The scientists credited the observed enlarged bandwidths for 2-D-ZnSb to the enhanced sp2 nature of honeycomb-structured layers with weakened interlayer interactions that formed the semi-metal. They used theoretical calculations to confirm that 2-D-ZnSb could be mechanically exfoliated into the bilayer to exist in an energetically stable form as a 2-D material, while the monolayer of 2-D-ZnSb was energetically unfavorable.
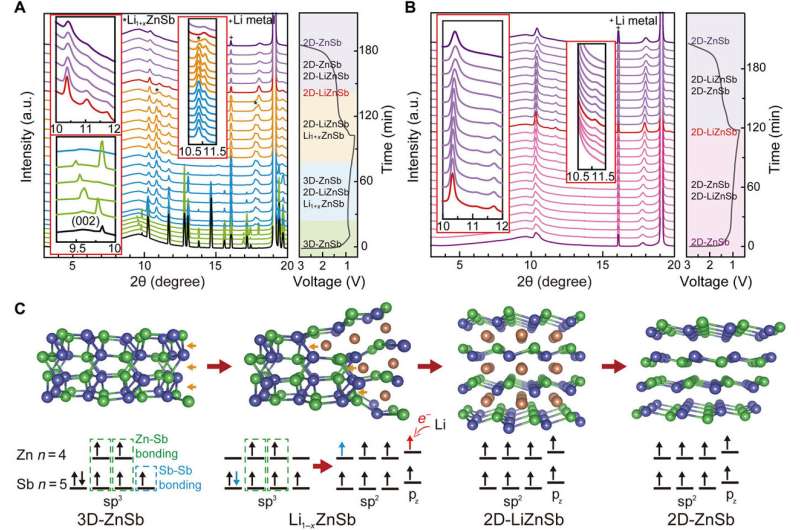
To demonstrate structural transformation of bidimensional ZnSb polymorphs during 2-D-LiZnSb formation, the scientists conducted - during the electrochemical reaction. They observed peaks corresponding to Li alloying of 3-D-ZnSb at pure 2-D-LiZnSb formation, followed by the final product of 2-D-ZnSb. During the electrochemical reaction, the Li atoms selectively penetrated into 3-D-ZnSb to break the Zn-Sb and Sb-Sb bonds. At the level of electron transfer, the hybridized bonding state changed from sp3 in 3-D-ZnSb to sp2 in 2-D-LiZnSb to form the puckered honeycomb lattice.
The outcome of Li alloying based 2-D-LiZnSb transformation yielded the 2-D-ZnSb product, which did not return to its 3-D form. Song et al. showed that once formed, the layered 2-D-ZnSb was a stable material with a honeycomb architecture, validating the stable bidimensional polymorphic transition. The scientists anticipate applications of the new material in sustainable alkali ion batteries.
In this way, Junseong Song and co-workers performed rigorous experimental and theoretical studies to demonstrate the creation of 2-D layered Zintl phases by manipulating the structural dimensionality. The new method is a first to establish the bidimensional polymorphic family in Zintl phases at ambient pressure, to allow new phase transformations as a general route of synthesis. This work provides a rational design strategy to explore new 2-D layered materials and unlock further properties of interest within materials, such as 2-D magnetism, ferroelectricity, thermoelectricity and topological states for further applications.
More information: Paul F. McMillan. New materials from high-pressure experiments, Nature Materials (2003).
Manish Chhowalla et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets, Nature Chemistry (2013). .
Kenneth S. Burch et al. Magnetism in two-dimensional van der Waals materials, Nature (2018).
Junseong Song et al. Creation of two-dimensional layered Zintl phase by dimensional manipulation of crystal structure, Science Advances (2019).
Journal information: Nature Chemistry , Science Advances , Nature , Nature Materials
© 2019 Science X Network




















