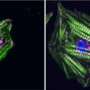
Folded and partially unfolded structure of talin subdomain. Credit: Vasyl Mykuliak, University of Tampere, Faculty of Medicine and Life Sciences;Vesa Hytönen,University of Tampere, Faculty of Medicine and Life Sciences
A study carried out as a collaborative approach between University of Tampere, Finland, and Imperial College London has shown that mechanically regulated proteins talin and α-catenin have stable intermediates during mechanical unfolding. The stable unfolding intermediates are formed by three α-helices.
In this study, a combination of steered molecular dynamics simulations, polyprotein engineering, and single-molecule atomic force microscopy was used to investigate unfolding of these proteins. Talin and α-catenin are α-helical proteins that pay a key role in formation of macromolecular complexes that enable cells to interact with the extracellular matrix or other cells.
Mechanical forces that are transmitted between the cell and its environment activate binding and regulate the functions of these scaffolding proteins at cell-extracellular matrix and cell-cell contacts. Thus, mechanical stability is a key feature in the regulation of functions of these structural scaffolding proteins. It was demonstrated that talin and α-catenin unfold through stable 3-helix intermediates, that represent biologically active states, and may allow recruitment of other binding partners.
The research was performed in collaboration between University of Tampere, Finland, and Imperial College London. Researchers at Tampere were responsible for computational work while researchers in London carried out the single-molecule atomic force microscopy experiments.
More information: Vasyl V. Mykuliak et al, Mechanical unfolding reveals stable 3-helix intermediates in talin and α-catenin, PLOS Computational Biology (2018).
Journal information: PLoS Computational Biology
Provided by University of Tampere