3-D structure of enzyme critical to creation of anticancer compounds in plants identified
Scientists identify 3D structure of enzyme critical to the creation of anticancer and antimalarial compounds in plants
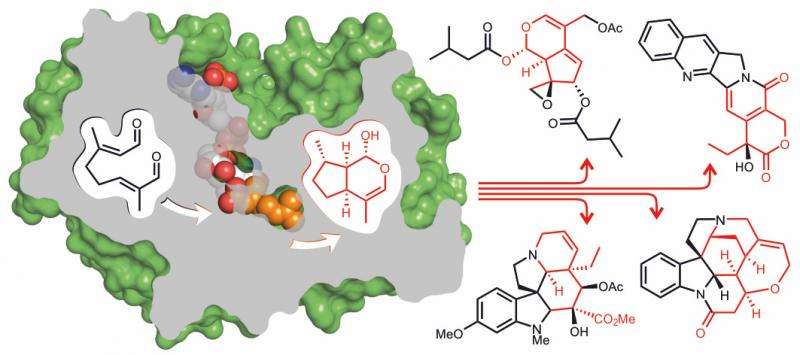
In a paper published today in Nature Chemical Biology, Professor Sarah O'Connor and Dr Dave Lawson have identified, for the first time, the 3D structure of the enzyme iridoid synthase responsible for a very specific form of cyclisation of monoterpenes which creates anticancer and antimalarial drugs.
The enzyme iridoid synthase plays a crucial role in the biosynthesis of a large class of plant natural products, the iridoids. Iridoids are the starting precursors for a large group of products such as the anticancer agent vinblastine, the antimalarial quinine and the active ingredient of catnip. Iridoid synthase generates the core of iridoid natural products by cyclizing a monoterpene precursor in a mode that is fundamentally different from other enzymes acting on monoterpenes.
The first gene of an iridoid synthase has only recently been discovered. In their paper they report the three-D structure of this enzyme which provides more detailed information on the mechanism of iridoid synthase.
More information: Structural determinants of reductive terpene cyclization in iridoid biosynthesis, Nature Chemical Biology,
Journal information: Nature Chemical Biology
Provided by John Innes Centre















