Optimizing atomic neighborhoods for speedier chemical reactions
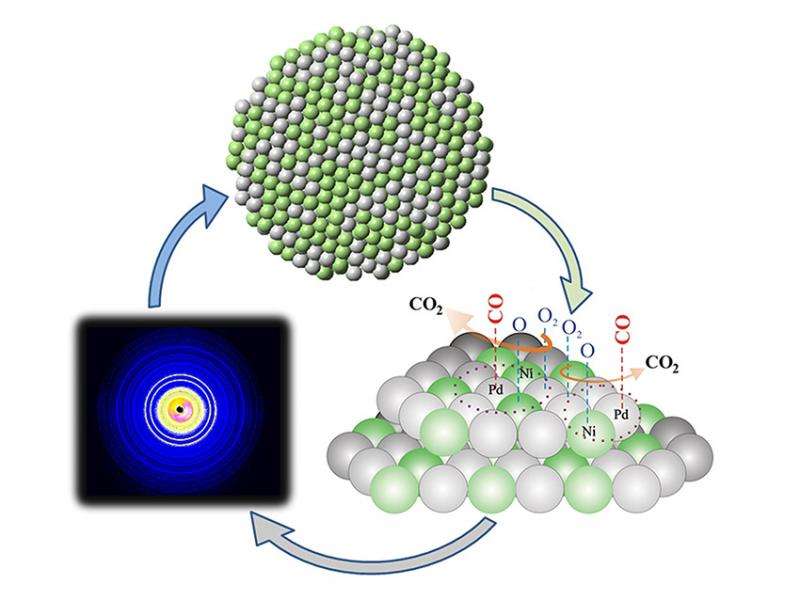
Scientists have discovered that for palladium-nickel catalysts, certain surface characteristics, measured at the atomic level, sped the creation of carbon dioxide from carbon monoxide. To reveal the optimal atomic neighborhood for surface chemical activity, high-energy x-rays were scattered by nanoparticles while they were exposed to a reactive chemical environment.
Catalysts accelerate chemical reactions and are essential to efficient industrial processes involved in energy production and pollution control. Employing in-operation tools to atomic-level interactions in palladium-based catalysts enhances the discovery and design cycles necessary for engineering low-cost, highly active, and stable catalysts.
Palladium-nickel nanoalloy catalysts have tunable parameters, such as particle size and atomic composition, that affect critical atomic-scale structural features. These key structural features include local bond distances and number of neighbors at surface sites, and are correlated with catalytic activity for the conversion of carbon monoxide into carbon dioxide. Surface sites with more atomic neighbors and longer first-neighbor separations were found to be more active catalytically than those with fewer atomic neighbors and shorter first-neighbor separations. This type of atomic detail has not been available by traditional studies and can aid the cycle of catalyst design by optimizing for structural parameters at the nearest neighbor level of an atomic environment. The atomic detail in a reactive environment was made possible by x-ray scattering techniques that involve high-energy x-rays and bright synchrotron x-ray beams able to penetrate sealed reaction chambers and resolve positions of atoms with high precision in minute amounts of nanoalloy particles. The work also demonstrated how various thermal and chemical environmental pathways alter the atomic neighborhood of nanoparticle surfaces.
More information: "Atomic-structural synergy for catalytic oxidation of CO over palladium-nickel nanoalloys." Journal of the American Chemical Society 136 (19), 7140–7151 (2014).
Journal information: Journal of the American Chemical Society
Provided by US Department of Energy




















